Photo by Freepik
The complex world of food supplement regulations in China can pose a significant challenge for foreign companies aiming to introduce their products to this large and ever-evolving market. The Chinese Food and Drug Administration (CFDA) has implemented stringent standards for food supplement registration, ensuring that only safe and effective products reach consumers. This article provides a comprehensive overview of the registration process and highlights key considerations for companies venturing into the Chinese market.
Deciphering Food Supplement Classifications in China
The initial step in the registration process involves correctly classifying the food supplement. In China, food supplements are categorized into two primary groups:
- Nutrition supplements: These products replenish essential vitamins, minerals, or other nutrients to supplement the diet. They are not intended to treat or prevent diseases.
- Functional health foods: These products are claimed to possess specific health benefits, such as boosting immunity, enhancing digestion, or alleviating stress. They undergo a more rigorous registration process compared to nutrition supplements.
Navigating Registration Requirements for Food Supplements in China
The registration process for food supplements varies based on the product classification. However, several general requirements apply to all food supplements:
- Company registration: The applicant must be a legally registered entity in China or have a duly authorized representative based in China.
- Product documentation: Detailed product information must be provided, encompassing product name, ingredients, formulation, manufacturing process, quality standards, and labeling.
- Safety and efficacy data: Comprehensive safety and efficacy data must be submitted to substantiate the product’s claims. This may include clinical trials, animal studies, and literature reviews.
- Testing reports: Testing reports from accredited laboratories must be provided to demonstrate that the product adheres to Chinese safety standards.
- Labeling compliance: The product label must comply with Chinese regulations and provide clear and accurate information about the product’s ingredients, usage instructions, and potential side effects.
Considerations for Foreign Companies
Foreign companies entering the Chinese market should consider the following additional aspects:
- Engagement of a local partner: Foreign companies must appoint a Chinese partner to manage the registration process and liaise with the CFDA. Chameleon Pharma Consulting Group can help you identify the ideal local partner.
- Document translation: All documents submitted to the CFDA must be translated into Chinese.
- GMP certification: The manufacturing facility for the food supplement must obtain Good Manufacturing Practices (GMP) certification from the relevant authority in the country of origin.
Timeline and Associated Costs for Food Supplements Registration in China
The registration process for food supplements typically takes 12 to 18 months to complete. The associated costs vary based on the product’s complexity and the testing required.
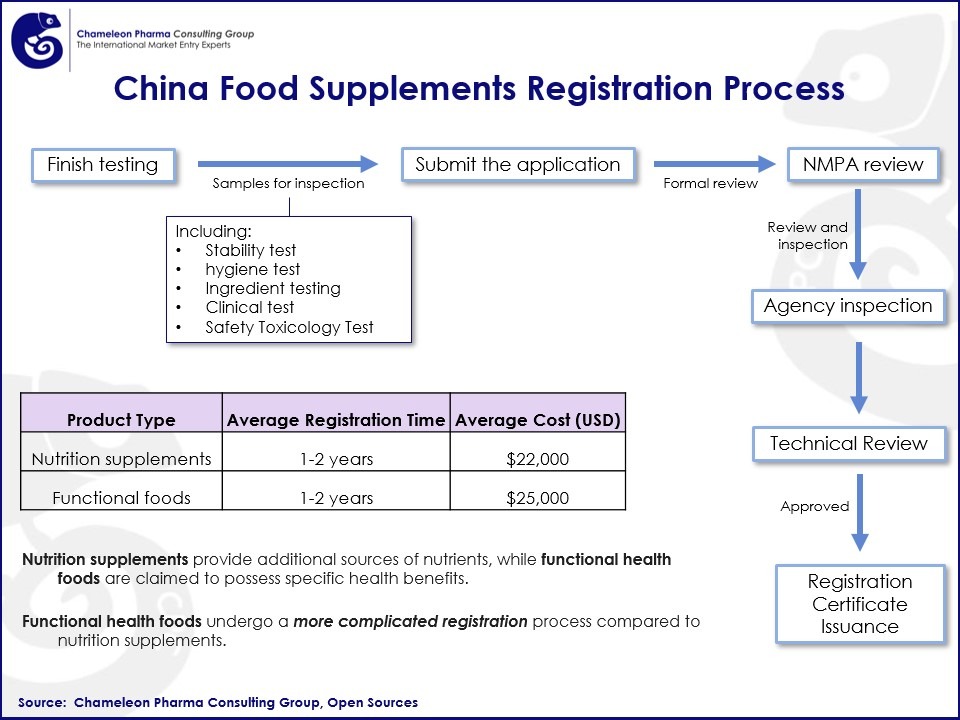
Figure 1. China Food Supplements Registration Process Flow Chart
The NMPA will meticulously review the application and make a decision. If approved, the NMPA will issue a registration certificate, allowing you to market and sell your food supplement in China.
Check out this article on the CPC website for further information about the Rx and OTC markets in China with the 2035 forecast, and this article for the Pharma & OTC Drug Registration guide. Stay tuned for the next parts regarding Medical Devices registration in China.
Chameleon Pharma Consulting Group has more than 20 years of experience in providing support to companies looking to enter or expand in international aesthetic and pharma markets. With the expertise amassed from more than 160 international projects and 22 experts around the world, CPC Group offers:
- Systematic product and country analysis;
- Systematic local partner identification;
- Regulatory and registration assistance.