Photo by Ksenia Yakovleva on Unsplash
China’s pharma and Consumer Health industry is rapidly growing, making it an attractive market for both domestic and international companies. However, the process of registering a medicine in China can be complex and time-consuming. This guide will provide you with an overview of the drug registration process in China, from applicant qualification determination to product safety and risk management.
Step 1: Applicant Qualification Determination
Before embarking on the registration process, it is crucial to ensure that your company meets the eligibility criteria set by the China Food and Drug Administration (CFDA). These requirements include:
- Legal Entity Registration: Your company must be a legally registered entity in China.
- Qualifications and Experience: Your company must demonstrate the necessary qualifications and experience to develop, manufacture, and market OTC drugs. This may include proven expertise in pharmaceutical research, manufacturing, and quality control.
- Compliance Track Record: Your company must have a clean track record of compliance with Chinese laws and regulations related to OTC and Rx products.
Step 2: IP Identification
Intellectual property (IP) protection is essential for safeguarding your company’s proprietary information and innovations. Prior to initiating the registration process, you must thoroughly identify and document all IP related to your drug, including patents, trademarks, and copyrights. Ensure that your company holds the necessary rights to utilize this IP.
Step 3: Summary Document of R&D Process and Results
Compile a summary document outlining the research and development (R&D) process undertaken for your drug. This document should provide detailed information on the drug’s ingredients, dosage form, intended use, and mechanism of action. Additionally, include data supporting the drug’s safety and efficacy.
Step 4: Product’s Technical Documents
Provide comprehensive technical documents for your drug, encompassing the following aspects:
- Detailed Description: A detailed description of the drug’s ingredients, dosage form, and intended use.
- Working Principle/Mechanism of Action: An explanation of the drug’s working principle or mechanism of action.
- Technical Specifications and Basis: The drug’s main technical specifications and the basis for these specifications.
- Raw Materials: A list of the drug’s main raw materials.
- Manufacturing Flow Chart: A manufacturing flow chart outlining the drug’s production process.
- Technical Index Inspection Methods: Methods for inspecting the drug’s main technical indexes to ensure quality control.
Step 5: Product Innovation Identification Documents
Substantiate your claims of product innovation by providing supporting documentation. Which will include:
- Academic Papers: Academic papers published in reputable journals that validate the drug’s efficacy and safety.
- Books and Literature Abstracts: Books and literature abstracts that provide relevant information on the drug’s therapeutic properties.
- Comparisons with Similar Products: Comparative analyses of your drug with similar products on the market, highlighting its unique advantages.
- Clinical Value Analysis: An in-depth analysis of the drug’s clinical value, demonstrating its potential benefits for patients.
Step 6: Product Safety and Risk Management Report
Conduct a thorough risk assessment to identify and evaluate potential risks associated with your drug. Prepare a detailed product safety and risk management report that outlines these risks and the measures implemented to mitigate them.
Step 7: Product Instruction Manual
Develop a clear and concise product instruction manual for your drug. The manual should be written in plain language and provide comprehensive information on the drug’s ingredients, dosage, potential side effects, and precautions for use.
Step 8: Other Documents
For foreign applicants, the following additional documents are required:
- Authorized Representative (AR): Foreign applicants must appoint an authorized representative (AR) who is a legal entity registered in China.
- Letter of Commitment from AR: The AR must provide a letter of commitment confirming their willingness to act as the applicant’s representative in China.
- AR Qualification Documents: Submit documentation demonstrating the AR’s qualifications and experience in handling drug registration matters.
- Statement of Authenticity: Provide a statement of authenticity confirming the accuracy and completeness of all submitted documents.
Step 9: CFDA Registration Process
Once all required documentation is prepared, initiate the formal registration process by submitting an application to the CFDA. The NMPA will meticulously review the application and make a decision. If approved, the NMPA will issue a registration certificate, allowing you to market and sell your drug in China.
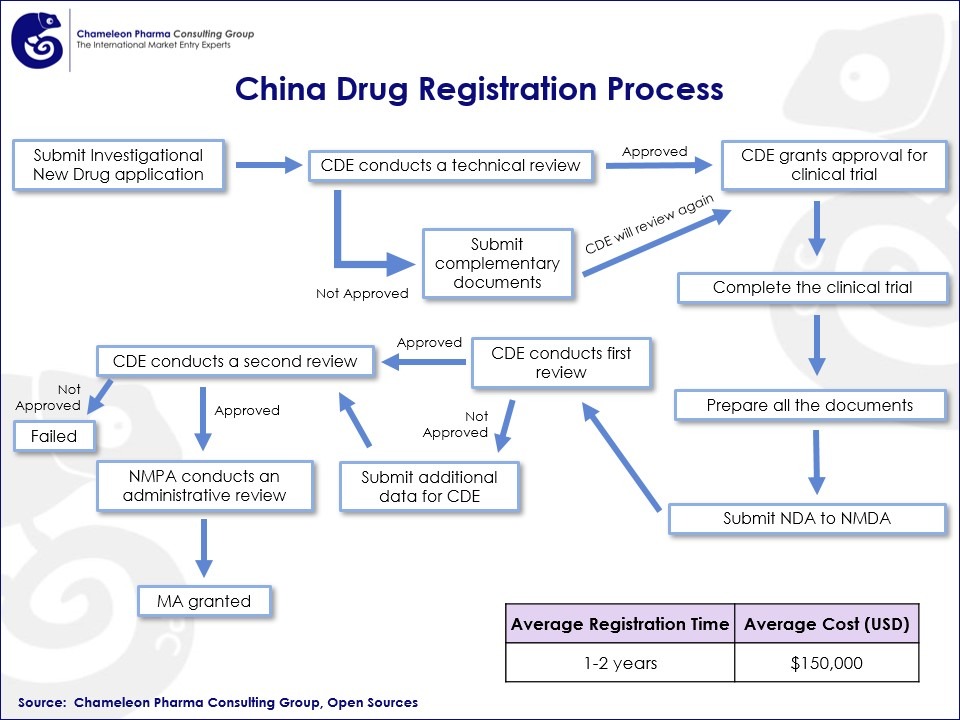
Figure 1. China Drug Registration Process Flow Chart
Check out this article on the CPC website for further information about the Rx and OTC markets in China with the 2035 forecast, and this article for the Food Supplements Registration guide. Stay tuned for the next parts regarding Medical Devices registration in China.
Chameleon Pharma Consulting Group has more than 20 years of experience in providing support to companies looking to enter or expand in international aesthetic and pharma markets. With the expertise amassed from more than 160 international projects and 22 experts around the world, CPC Group offers:
- Systematic product and country analysis;
- Systematic local partner identification;
- Regulatory and registration assistance.




