Featured picture: Photo by Mockup Graphics on Unsplash
China’s recent regulatory updates reflect an effort to harmonize with international standards of the International Medical Device Regulators Forum, along with the regulatory frameworks of the United States and the MDR in Europe. This new regulation allows foreign manufacturers to easily register their Class I medical devices in China.
Medical Devices Registration in China
Medical devices in China are categorized into three categories based on their potential risk to patients:
- Class I: Low-risk devices, for which routine administration is adequate for safety and effectiveness.
- Class II: Moderate-risk devices, for which further control is required to ensure safety and effectiveness.
- Class III: High-risk devices, with life support, and sustenance functions, including those that pose a potential threat to patients’ health or are implanted into the human body.
The entity in charge of reviewing medical device registration dossiers in China is the Chinese Medical Device Evaluation (CMDE), under the National Medical Products Administration (NMPA).
In the past years, the NMPA changed considerably the regulation for registration of medical devices in China:
- Class I medical devices are no longer subject to product registration, but to product listing, which is a simplified process.
- The new Clinical Evaluation Exempt Catalog presents a list of 2025 Class II and Class III medical devices that are exempted from the Clinical Evaluation Report. This list includes devices such as Medical Air Compressors, Breast Tissue Markers, and Venous Blood Containers, among others.
Listing Process for Imported Class I Medical Devices in China
To be able to distribute their Class II and Class III Medical Devices in China, manufacturers need to obtain the Medical Device Registration Certificate.
Manufacturers of Medical Devices Class I are not required to undergo type testing, clinical evaluation, or comprehensive risk management procedures. Overseas manufacturers must appoint a Chinese Legal Representative, a licensed entity in China, to act as their legal agent and handle all regulatory interactions with the NMPA. Chameleon Pharma Consulting Group can support you in finding your local representative.
For the Record of Importation of Class I Medical Devices, the following documentation must be provided:
- Information of the Chinese Legal Representative
- Proof and information of production
- Proof that the product is on the market in the home country of the legal manufacturer or production site
- Product Technical Requirement (PTR)
- Testing report
- Instructions for use (IFU) and label
- Declaration of conformity
Listing a Class I medical device in China incurs no fees. The review process typically extends over one month. Furthermore, the Notification of Record Number issued upon completion of the process does not have an expiration date.
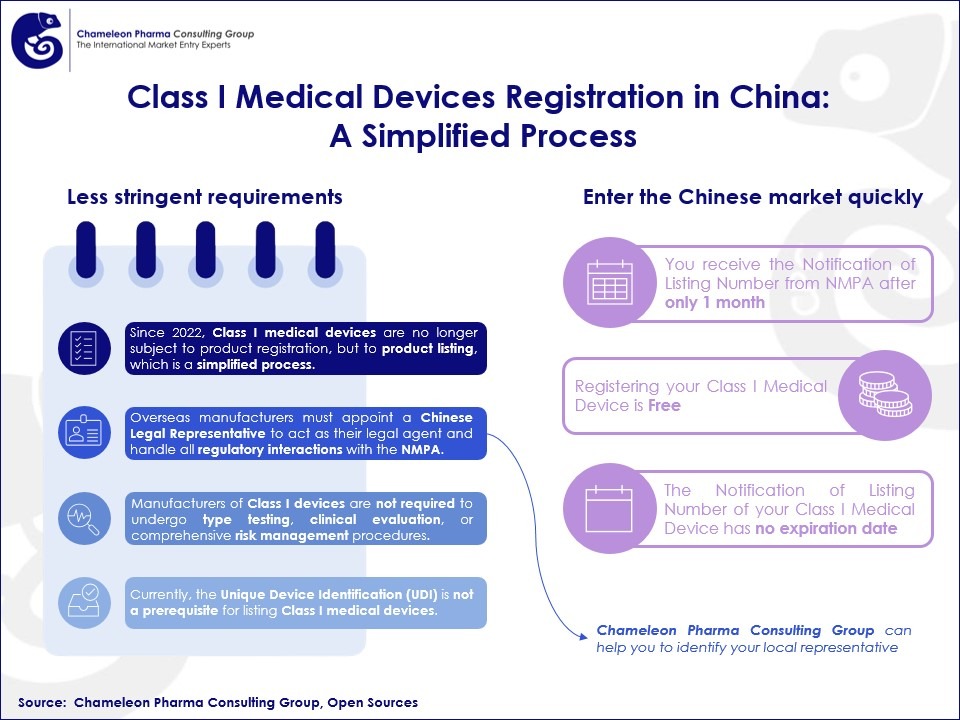
Figure 1. Class I Medical Devices Registration in China: A Simplified Process
By following these steps, you can get your Class I medical device successfully listed in China. Check out this article on the CPC website for further information about the Rx and OTC markets in China with the 2035 forecast, and this article for the Pharma & OTC Drug Registration guide. Stay tuned for the next part regarding registration of Medical Devices Class II and III in China.
Chameleon Pharma Consulting Group, with its extensive network of Chinese regulatory experts, would be delighted to support you in this process. We have long-term experience in handling registration and variations for more than 20 years. Contact us today with your individual questions!