Feature picture: Photo by Jair Lázaro on Unsplash
Get The Important Insights!
The registration and GMP certification of medical devices are critical steps for manufacturers aiming to enter the markets of Russia and the Eurasian Economic Union (EAEU). The new EAEU rules will become mandatory after December 31, 2025, so companies can still choose between the registration in Russia and EAEU. In this article, we will highlight the necessary steps in each case and describe the distinctions between the procedures in Russia and the EAEU.
Recent Regulatory Changes in Russia and EAEU
Recently, both the Russian and EAEU medical device markets saw significant developments and regulatory changes. Initially, the authorities planned to complete the transition to the mandatory registration of medical devices according to the EAEU rules by January 1, 2022.
However, the Council of the Eurasian Economic Commission (EEC) officially extended the national registration system until December 31, 2025. Therefore, manufacturers and distributors can choose between the national and EAEU registration procedures for their medical devices until 2026.
The National Procedure of GMP Certification & Registration of Medical Devices in Russia
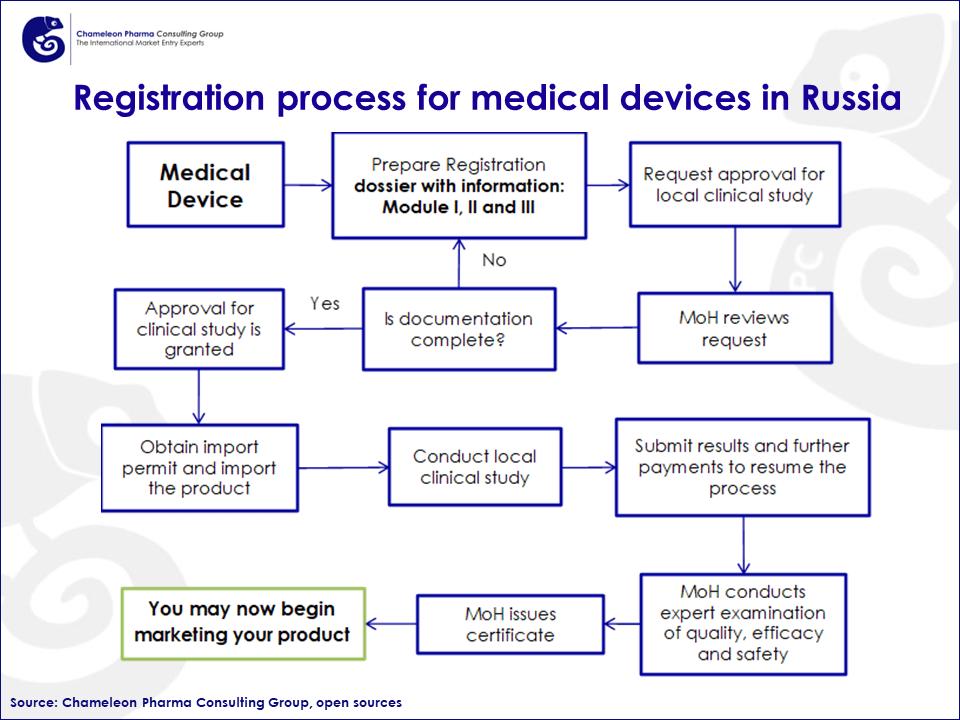
The steps for registering medical devices in Russia are as follows:
- Document Preparation: Due to the large number of medical products, their differences, nuances and legislative amendments, the list of required documents may vary. The most effective way to ensure a successful registration might be to consult Medical Device experts who are aware of the local regulatory nuances like Chameleon Pharma.
- Testing in accredited laboratories:
- Single-step process for Risk Class 1 products and in vitro diagnostic products.
- Two-step process for Risk Class 2a, 2b, and 3 products.
- Submission of the registration dossier to Federal Service for Surveillance in Healthcare (Roszdravnadzor)
- Approval and receipt of a registration certificate.
One of the most significant changes in the national procedure concerns the GMP-certification procedures. Starting from mid-2023 Class IIa sterile, Class IIb, or Class 3 medical devices already registered through national legislature will need to have an onsite GMP-inspection rather than using a legalized ISO 13485 certificate, as was allowed under previous Russian requirements.
GMP Certification & Registration of Medical Devices in the EAEU
The new EAEU rules will become mandatory after December 31, 2025. Therefore, the registration under the rules of the EAEU will remain voluntary until the beginning of 2026. There are several key points to take into account before opting for the EAEU registration:
- Regulatory changes: The EEC Board defined several new criteria for classifying products as medical devices. For example, primary packaging of medicines (cans of medical spray) or furniture are not subject to registration in the EAEU. This requires a closer analysis of products that are subject to registration.
- Stages and Order: The registration process in the EAEU involves several stages, including document preparation, testing, dossier submission, quality, safety, and effectiveness assessment, production inspection, expert opinion coordination, and issuance of a registration certificate, which needs to be later harmonized by the EAEU states of recognition.
- Price: The cost of EAEU registration includes documentation development, test samples, testing, quality management system implementation, production inspection, state duties, translations, notary services, and bank commissions. The exact expenses depend on various factors.
- Required Documents: The necessary documents for EAEU registration depend on the device’s risk class, which requires a thorough analysis by the company or a local expert, like Chameleon Pharma.

Where to register your medical devices – in the EAEU or Russia?
Contact the CPC experts today to receive full details about the main differences between the process of registering medical device in Russia and the EAEU!

Photo by Slava Taukachou on Unsplash
Choosing between the two procedures requires a comprehensive analysis and a clear understanding of the differences. On the one hand, the national rules are easier to follow since they have been tested over time. They would be more suitable for companies wanting to enter the market in a quick and non-expensive way. On the other hand, the new EAEU rules may seem complicated at first glance, but offer more benefits in the long run, especially taking into account the access to the entire EAEU market on an indefinite basis.
Cooperating with a local partner is usually the first choice for foreign medical device companies entering the Russian or EAEU market. Chameleon Pharma Consulting Group is here to support you with the initial regulatory process, GMP Inspection, Variations and the systematic identification of the right local partner & ideal product.
Contact us at service@chameleon-pharma.com !