Feature picture: Photo by Fajruddin Mudzakkir on Unsplash
Get The Important Insights!
By 2035 the Rx and OTC Pharma market in Indonesia is estimated to rise to a valuation of 27.16 billion USD owing to a substantial CAGR of 8.75%. Continue reading to discover the opportunities of this growing Asian healthcare market.
Growing Indonesian Consumer Health & Rx. Pharma Market
Being the fourth most populated country in the world with an increasingly aging population, Indonesia is a growing – and therefore appealing market for Rx and OTC products. It is estimated that by 2035 the sales of Rx and OTC will be valued at approximately to 27.16 billion USD with a CAGR of 8.75%.
There are several driving factors behind this rapid growth:
- Healthcare is high on the public agenda in Indonesia, which is reflected in their substantial health insurance system (JKN). In 2022, 86% of the population was registered in the system which has increased the demand for advanced medical equipment and supplies.
- By 2025, it is expected that the elderly will make up 12.5% of Indonesia’s population. This is a 45% increase from 2015. A more elderly population invariably increases the demand for food supplements, VMS, in addition to the essential Rx and OTC drugs.
- The growing urban population will also increase the demand for various types of medicines, MD and beauty products, as the health standard of the population improves.
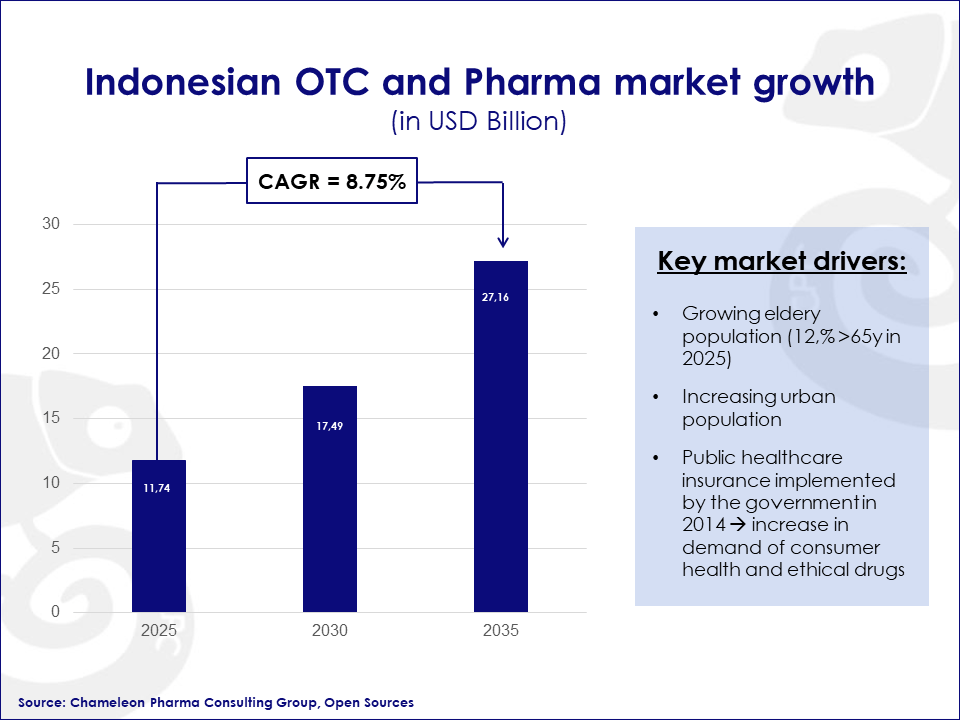
Indonesia’s OTC & Pharma market growth projection
Licensing and Distribution
Indonesia relies heavily on imported medicine, medical equipment and supplies to meet local demand. Most companies will find it beneficial to choose a local distributor to assist with product registration, import permits, and the logistics of importing and distributing their devices in the local market.
The registration of pharma products is done via the Food and Medicine Supervisory Board (BPOM).
- If pharma products already have a CTD certificate, the registration process is faster. The CTD certificate must follow the rules of the ASEAN CTD standard.
- The application for marketing authorisation must be requested via the Indonesian government’s Online Single Submission (OSS) system.
- Any imported medicine must be registered by a domestic pharmaceutical producer that has obtained written consent from the relevant foreign pharmaceutical producer. This written consent is not needed, if the domestic pharmaceutical producer is affiliated with the foreign pharmaceutical producer. If the registration is approved, the applicant will receive a digital distribution licence through the OSS portal.
In order to distribute OTC and Rx products, companies need to have the Medical Device Distribution Certificate (IPAK) from the Ministry of Health.
There are two important things to note when exploring new consumer health and pharma opportunities in Indonesia. First, if a product can be manufactured locally, the Indonesia government prohibits the importation of a similar product. However, if the product is proven to include or require innovative technology that local manufacturers cannot replicate, the product may have the opportunity to enter the Indonesian market. Second, OTC and pharma products will have to be halal certified in the future and products that originate from haram (forbidden) substances will require to be labelled accordingly.
Photo by Nick Agus Arya on Unsplash

How can Chameleon Pharma support you in expanding your business in Asia?
The Indonesian market relies heavily on the import of OTC and Rx drugs, opening the opportunity for international players to participate. This is the right time for your company to expand its business scope beyond your home country. However, kindly note that choosing the right local partner is the most crucial key to a successful launch. With more than 25 years of experience in emerging markets – especially Southeast Asia, Chameleon Pharma Consulting Group can support you in identifying your perfect match!




