On July 17th 2020 it was time to present our to clients how to easily access the Russian OTC & Rx market. Our CPC experts detailed the Consumer Healthcare and Pharma drugs registration process and how to acquire the GMP certification.
If you missed the first session, our CPC team is currently conducting a Pharma Insiders Webinar, where our experts present to our clients how they can easily access pharma markets all over the world.
Our second free webinar was about the Russian OTC & Rx drugs market and why it is important to proceed with drug registration before the end of the year 2020, since the regulations will change and make the process even more complex and difficult.
Russia is one of the biggest Consumer Healthcare & Pharma markets in the world and a country with more than 145 million people, showing a good potential for new and innovative products.
Followed by participants’ questions, our webinar covered:
- The potential in the Russian and EAEU Region for Consumer Healthcare and Pharma drugs;
- What is required by the Russian MoH when it comes to OTC and Rx registration in Russia;
- What are the costs and time of the registration process for OTC and Rx drugs in Russia;
- How simple are the clinical studies in Russia and how to handle them;
- How to comply with GMP requirements; and
- How generics and original drugs differ on the registration process.
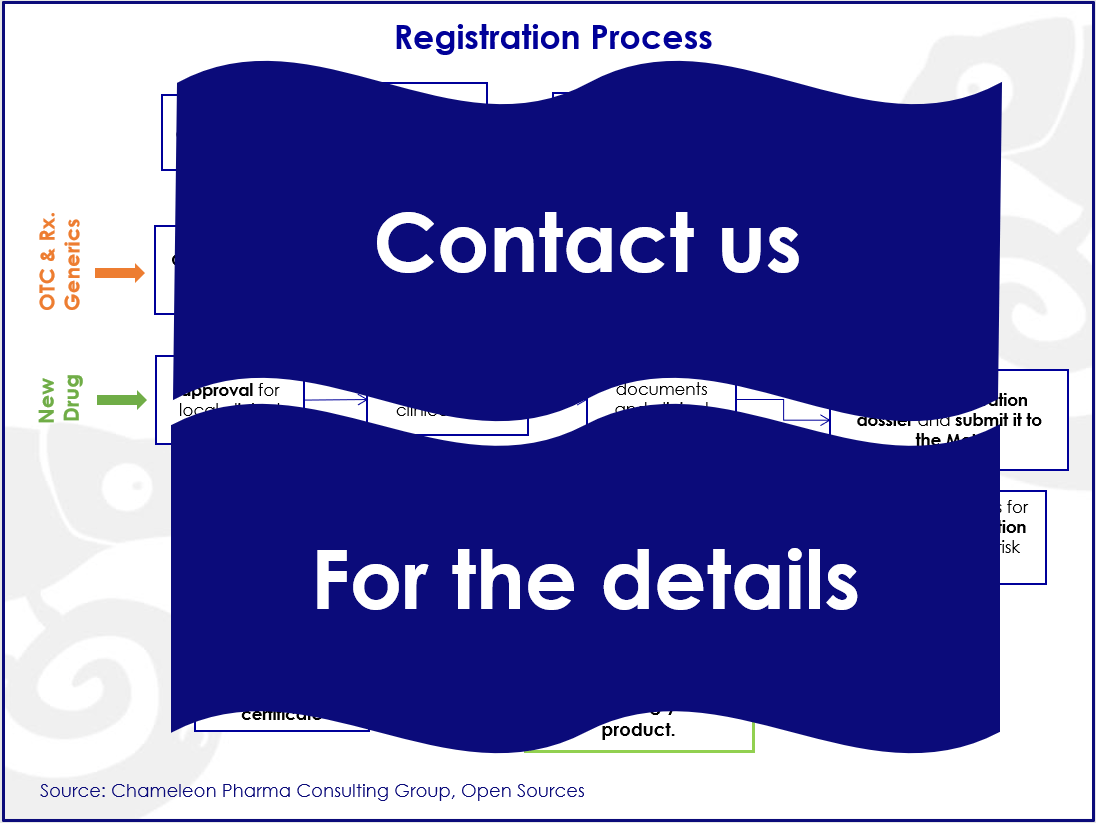
Registration Process of OTC and Rx drugs in Russia
Other webinars are being prepared and will be presented soon: Brazil, EU Region and Ukraine. Stay tuned on our website and social media so you don’t miss any other opportunities!
Also, if you would like to know more about registering your product in Russia, our experts are more than ready to support you and to answer your questions! Just call us or contact us via e-mail.
Featured Image: Photo by Dušan veverkolog on Unsplash




