Photo by Viktor Hesse on Unsplash
In the rapidly evolving pharma & OTC landscape of the Eurasian Economic Union (EAEU), obtaining a Good Manufacturing Practice (GMP) certificate is no longer just a regulatory hurdle: it is a strategic gateway to a market of over 180 million people. However, the path to compliance is complex, and the stakes for manufacturing sites are high.
The EAEU GMP Landscape: A Unified but strict system
The EAEU GMP framework, primarily governed by Decision No. 83, has been 100% unified since January 2026. While these rules are largely harmonized with EU GMP standards, they maintain specific local requirements that manufacturers must address. Inspections are carried out by a Member State’s pharmaceutical inspectorate, such as Russia’s MINPROMTORG, and can be conducted either on-site or via a remote format.
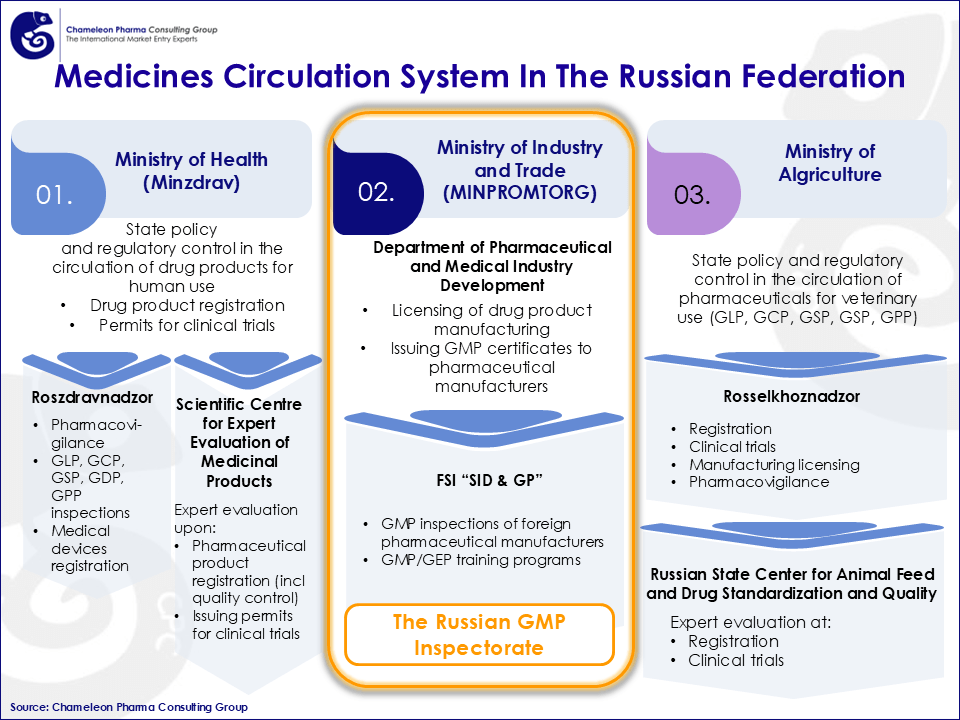
Figure 1. Medicines Circulation System In The Russian Federation
Under this unified system, a standard EAEU GMP certificate is typically valid for three years, though this period may be shortened based on a risk-based assessment. Compliance remains essential, as the identification of major or critical non-conformities during an inspection can lead to the immediate refusal of a certificate or the suspension of existing marketing authorizations.
Why is an EAEU GMP Pre-Audit Necessary?
To minimize the risk of deficiency letter, CPC recommends a comprehensive EAEU Pre-Audit conducted 1 to 3 months before your scheduled inspection. This process is designed to mirror the actual inspection by Minpromtorg to ensure your plant is truly “inspection-ready”, thus saving a significant amount of time and effort.
The CPC EAEU GMP Pre-Audit Process Includes:
- GAP Analysis: A thorough review of your Pharmaceutical Quality System (PQS), Quality Control (QC), and Site Master File (SMF) against EAEU regulatory requirements.
- On-site or Remote Inspection: A 3–4 day mock inspection covering both documentation and a tour of your manufacturing site.
- CAPA Support: Following the audit, we provide a detailed report of non-conformities and work with your team to develop a robust Corrective and Preventive Action (CAPA) plan.
- Inspector Coaching: We prepare your staff for the specific questioning styles and focus areas of EAEU inspectors.

Figure 2. CPC Pre-Audit Process for EAEU GMP readiness
Beyond the Audit: Comprehensive EAEU GMP Support from CPC
Our services extend through every stage of the certification timeline, which can take anywhere from 3 to 6 months or longer depending on application volume.
Our end-to-end support includes:
- Dossier Submission: Preparing and submitting the inspection application to the Ministry of Industry and Trade.
- Negotiation & Hosting: Handling all communications, payments, and date settlements with the Russian inspectorate on your behalf.
- Post-Inspection Facilitation: Assisting with the implementation of CAPAs after the official inspection and delivering the final decision to your company.
- Deficiency Letter Support: If you have already received a refusal or deficiency letter, our network within the MoH and Minpromtorg allows us to help you rectify the situation and secure approval.
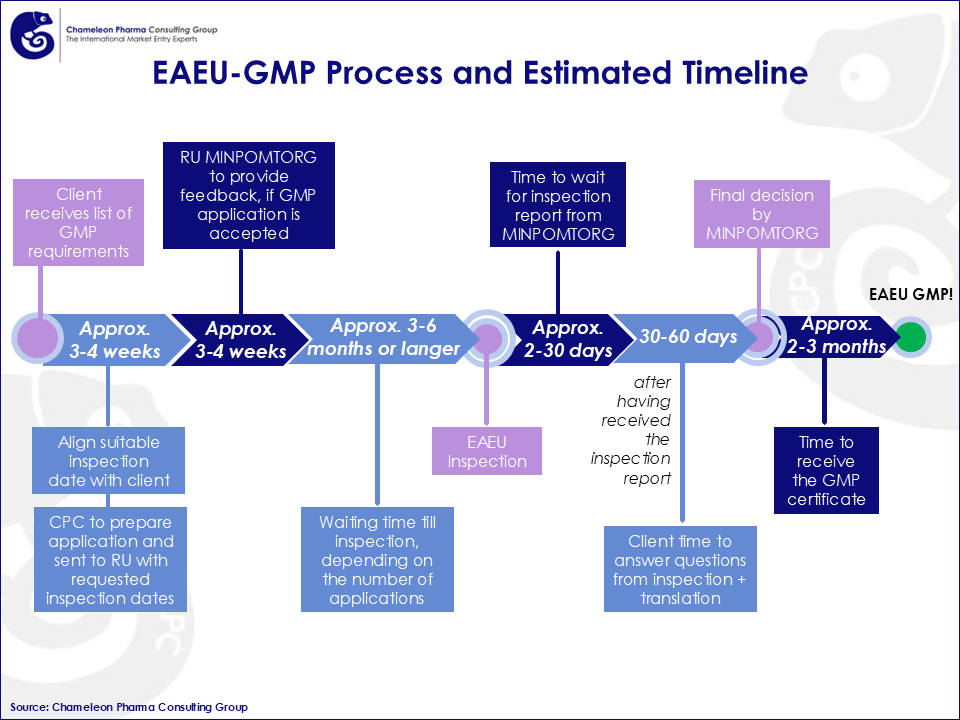
Figure 3. EAEU-GMP Process and Estimated Timeline
Is Your Manufacturing Site Ready for the EAEU Market?
At Chameleon Pharma Consulting (CPC), we have supported over 80 successful EAEU and Russian GMP inspections. Our experience shows that the difference between a seamless approval and a costly refusal often lies in the quality of preparation before the inspectors ever set foot on-site.
CPC has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




