Australia TGA has introduced new guidelines for the regulation of substances that may be used in listed OTC and pharma products. The new guidelines replace the old ARGCM V8.0 Part C and contain items such as eligible substances, application categories, timeframes, fees and market exclusivity among other topics.
So let’s answer the first question: Which substances are allowed for use in listed Consumer Health and pharma products?
Those are substances not prohibited for import as well as substances that are not subject to the conditions of a schedule to the Poisons Standard. They are organized in the following 4 levels:
- IN1
- IN2
- IN3
- IN4
The lower the level of the application, the easier the process, meaning less supporting information and less time is required for the application. In the table below you can find the curtail application requirements in order to get a successful application opportunity.
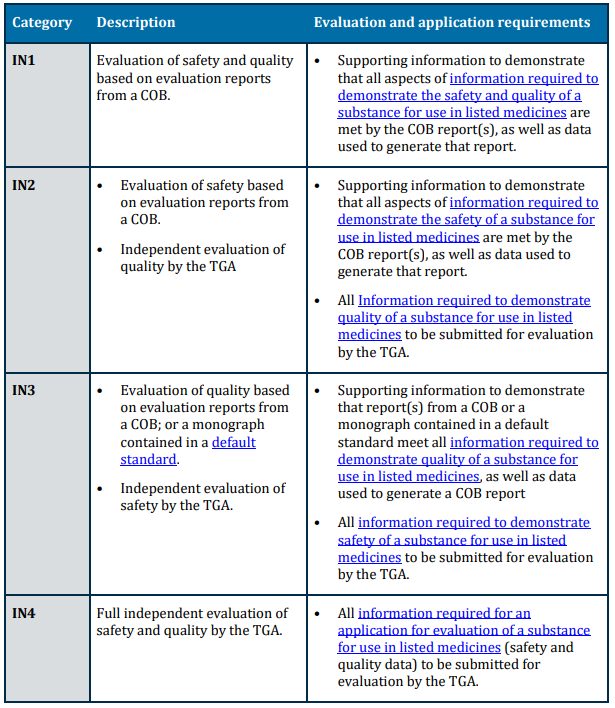
Table 1: Application categories for evaluation of substances – Source: www.tga.gov.au
Timeframes and fees
The preliminary assessment timeframe for all substances is 40 days, but the higher the level of the new Australia TGA guideline the more evaluation days are required varying between 70 and 180 days depending on the application level.
The application process for evaluation of substances regarding the use in listed OTC and Pharma requires the following 6 phases:
- Phase 1: Pre-submission phase
- Phase 2: Lodgment of application and payment of application fee
- Phase 3: Preliminary assessment of the application
- Phase 4: Evaluation
- Phase 5: Recommendation
- Phase 6: Finalization
Just give us a call for more information you do require regarding your own products registration in Australia.
Featured Image: Animated Heaven – Checklist



