Get The Important Insights!
Mexico represents the second largest OTC and pharma market in Latin America, and one of the most profitable around the world. Its drug, food supplement, cosmetic and medical devices‘ registration method could be seen as a perturbing step to enter its market.
The main aim of this article is to bring some clarity to the registration process regarding medication and, more specifically, new molecules and generic drugs in Mexico.
COFEPRIS (The Federal Commission for the Protection against Health Risks) – the Mexican authority that regulates the registration and safety of pharmaceutical products, defines “new molecules” as all medicines containing:
- New active ingredients;
- Completely new drug with limited clinical evidence;
- New compounds;
- New indication or new dosage forms.
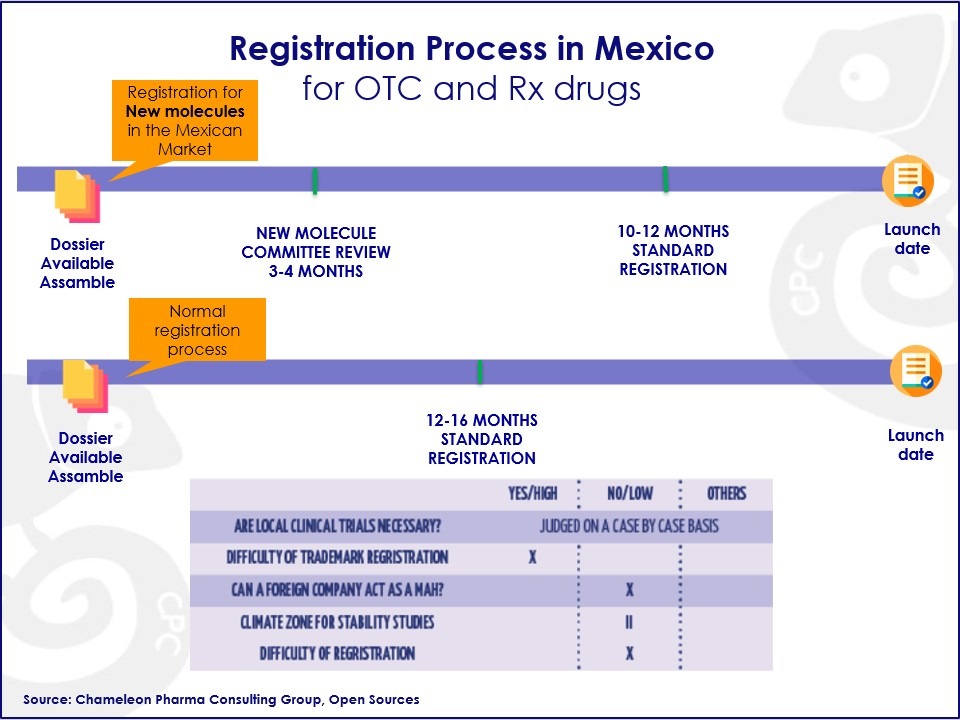
Figure: Registration Process in Mexico for OTC and Rx Drugs
The registration of the medicines that fall into this category is longer and more complex. It has to be reviewed by the Committees for New Molecules who will determine if the molecule can be considered new.
Moreover, having the product already registered in the USA, Canada, EMA, Switzerland, or Australia can be an important advantage and may qualify your molecule to go through the Equivalency Review Process. Registering a generic drug would mean going through a less complex procedure.
In fact, the product simply needs to comply with the equivalences set by the COFEPRIS and have a dossier and documents regarding the quality, bioavailability, and other required documents ready for the registration process. In both cases, after submitting the enquiry, the COFEPRIS will decide whether to register the product.
Since the middle of August 2021, Mexico has accepted English dossiers without the necessity of Spanish translation. However, in reality, COFEPRIS employees have been overwhelmed understanding and judging English clinical trials and other complex regulatory details. We expect that by the 5th of May 2022 or earlier, Spanish dossier translation will become mandatory again. Companies wanting to introduce products in Mexico should be quick in order to save between 30.000 – 40.000 US$ in the translation process.

The regulatory framework may vary from country to country and this may present a challenge for all companies, especially in those expanding into emerging markets. For this reason, we are ready to support you and your business with all your regulatory-related needs, such as GMP certificates, product registration, variations and other such matters.
We have more than 20 years of pharmaceutical expertise in emerging markets such as Brazil, China, Mexico, Russia, in addition to the European Union.