Photo by Andrey Kukharenko on Unsplash
Get The Important Insights!
Are you interested in maintaining & expanding your business into the Eurasian Economic Union (EAEU) region including Russia? Read some insights about how to obtain the EAEU GMP certification for Pharma & OTC drugs.
The former Commonwealth of Independent States (CIS) region is located in Eastern Europe and Asia and was established by former member states of the Soviet Union. The region’s market is considered to be an emerging market, and several countries–namely Kazakhstan and the Russian Federation– have recently been recognized as fast-growing consumer healthcare and pharma markets. In terms of the value of the OTC and Rx. market, the Russian Federation ranks fifth among the evaluated European countries, after Germany, France, Italy, and the United Kingdom. These, amongst other advantages, make the CIS region attractive to growing OTC and pharma companies. The drug GMP certificate is one of the most important documents allowing the production of medical products in this region. A correct interpretation of pharma and OTC drug GMP requirements is critical to the success of companies, but often presents them with extremely difficult challenges.
The Eurasian Economic Union (EAEU) is an international organization for regional economic integration with international legal personality and established by the Treaty on the Eurasian Economic Union of May 29, 2014. The EAEU ensures the freedom of movement of goods, as well as services, capital, and labor, and the implementation of a coordinated, agreed, or unified policy in the sectors of the economy.
Currently, the EAEU includes five countries: the Republic of Armenia, the Republic of Belarus, the Republic of Kazakhstan, the Kyrgyz Republic, and the Russian Federation.
The EAEU was created for the purpose of comprehensive modernization, cooperation, increasing the competitiveness of national economies, and creating conditions for stable development in the interests of improving the living standards of the population of the member states. The population of the EAEU is around 184 million people. The aggregate GDP is 1,838 billion USD, and is 10th in the world.
In order to create a common market in the territory of the EAEU, uniform principles and rules for registration and circulation of medicines; technical regulations for medical devices, food supplements, and cosmetics were introduced in accordance with the Treaty on the Eurasian Economic Union of May 29, 2014.
By 2033 the value of the global OTC and pharma market is expected to be around USD 2.000 billion. The share of the EAEU market in the world will be 5.1%, which is more than USD 60 billion. The fundamental document for creating a single market for medicine is the Agreement on Unified Principles and Rules for the Circulation of Medicines within the Eurasian Economic Union dated December 23, 2014. Unified rules for the examination of medicines, for GMP, GVP, GCP, GDP, GLP, inspection, requirements for drug labeling, and many other legislative acts were approved.
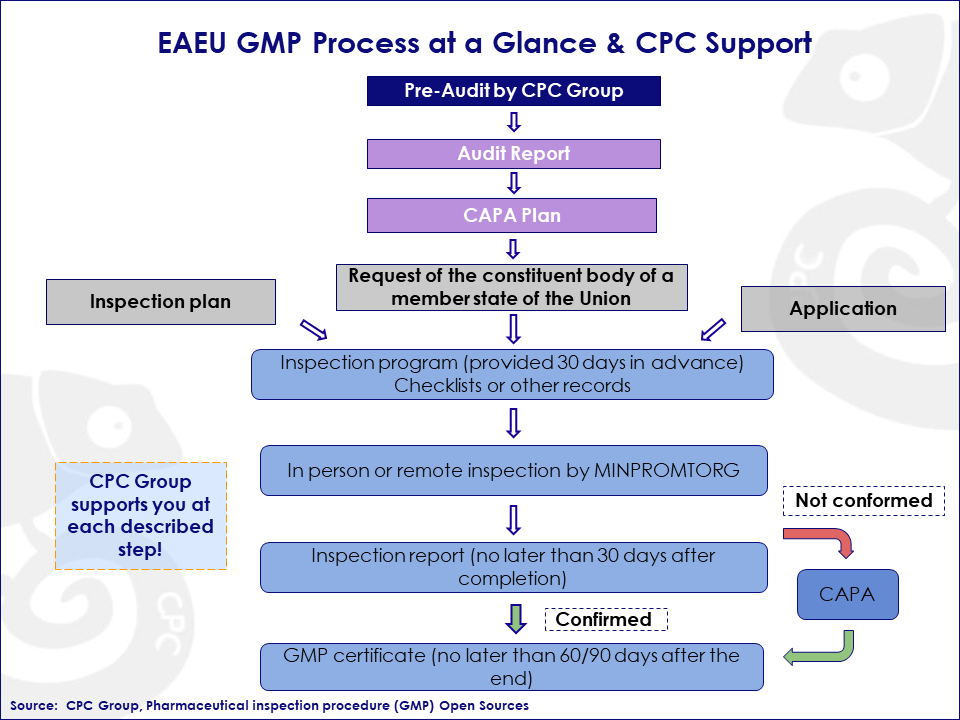
Figure: GMP Process at a Glance
Inspection is carried out by the pharmaceutical inspectorate on the basis of: an inspection plan, an application for inspection, or at the request of an authorized body of a member state of the Eurasian Economic Union. An inspection would be necessary for the purpose of licensing, registration, or investigations related to the quality of medicinal products for example. The inspection process is conducted in accordance with the production of the medicinal products inspection program, according to Appendix No. 1 (of the rules for conducting pharmaceutical inspections in the EAEU region).
The Russian Ministry of Industry and Trade (MINPROMTORG) provides a state service issuing a certificate of conformity for non-resident drug manufacturers, with the requirements of the GMP rules of the Eurasian Economic Union.
At the opening meeting, the inspection protocol is introduced to the inspected party, the objectives and scope are announced, and its program and schedule are specified.
Given the current circumstances, the inspection may be in person or remotely via video conference.
During the inspection, the members of the inspection team carry out:
- inspection of checked objects,
- familiarization with documentation and records,
- interviewing the responsible persons of the inspected entity
- monitoring activities in the workplace
The information received is entered into a checklist or other forms of work records.
If necessary during the inspection, samples of materials or products are taken, which are sent for testing to an authorized testing laboratory.
The lead inspector draws up a report no later than 30 calendar days from the date of completion of the inspection.
If inconsistencies have been identified, the inspected entity sends a response to the pharmaceutical inspectorate with the attachment of a corrective and preventive action plan (CAPA) and a report on its implementation no later than 30 calendar days from the date of receipt of the report.
Within 30 calendar days from the date of receipt of the specified response, the lead inspector evaluates the information contained in it and, if necessary, performs another (control) inspection.
Subject to the elimination of all critical and significant inconsistencies, the EAEU GMP Certificate is issued.
Due to the COVID-19 pandemic, more and more remote inspections are carried out.
The EAEU GMP inspection of drug production is becoming an integral part of the process of placing a drug on the Russian and CIS market. Our company’s experts are actively monitoring the legislation of the Russian Federation in the field of regulation of the circulation of medicines in Russia and the CIS countries.
We at Chameleon Pharma Consulting can and will support you at every step of the EAEU OTC and pharma GMP certification process. If you have detailed questions regarding your products’ registration and the pharma GMP process, cost, time etc., please kindly contact us.
Changes as of January 1st 2023 and important deadlines
The EAEU GMP certificate has become mandatory for Russia as well as for the other EAEU member countries. All companies selling on the Russian market and neighboring countries have to bring their regulatory dossiers in line with the new EAEU guidelines by the 31st of December 2024.
Moreover, all OTC and Rx drug players have to successfully gain the EAEU GMP certificate by the 31st of December 2024.
The EAEU GMP inspection process, as well as the update of the regulatory dossiers, is a big challenge for many importing pharma companies.
It is highly recommended for importing OTC and pharma companies to conduct a pre-EAEU audit on their own pharma plants. This will highlight the possible gaps and issues regarding the new EAEU GMP guidelines and thus save a lot of money and time, before the official inspectors from MINPROMTORG will review the foreign pharma factory.
How CPC can support you during the EAEU GMP process?
We at CPC support our clients with more than 20 years of experience regarding EAEU GMP audits and inspections. Furthermore, CPC is supporting its clients to bring regulatory dossiers in line with EAEU GMP standards. Just give us a call to discuss your specific product situation.
“Many of our clients have raised a question, if they can also receive the EAEU GMP certificate from Armenia instead of Russia. Theoretically, this should be possible. On a practical basis, we do not believe Russia will be ok not receiving the regulatory and GMP fee´s. Having this in mind, we have seen many companies failing the EAEU GMP audit via Armenia. Therefore, we strongly recommend to submit the inscription via Russia in case you plan to sell the product there.”




