Photo by Yuri Krupenin on Unsplash
Understanding Germany’s pharma tender system is essential for market success. With strict rebate laws (§130–131 SGB V), a regulated value chain, and tender models shaping access, manufacturers must navigate legal, pricing, and supply challenges to compete in Europe’s biggest market.
Overview of the German Market
Germany is Europe’s largest pharma market and the fourth largest worldwide, after the US, China and Japan. Driven by trends such as demographic change, a rise in chronic diseases and an increasing emphasis on prevention and self-medication, the pharma market is growing rapidly. The German total pharma & OTC sales are projected to expand from USD 78.04 billion in 2030 to USD 153.98 billion by 2040 with a CAGR of 7.03%.
Pharma Tender Process in Germany
In Germany, pharma tendering (Ausschreibung) is a key instrument used by statutory health insurance funds (GKV) to control drug costs and ensure reliable supply. Manufacturers compete by offering discounts (rebates) in exchange for preferred reimbursement and dispensing status. A core distinction exists between open-house tenders, where all qualifying manufacturers can enter under identical conditions, and closed tenders, which involve exclusive contracts awarded to one or a few bidders offering the most favourable terms. These rebate agreements (Rabattverträge) are central to the system and directly shape market access and competition.
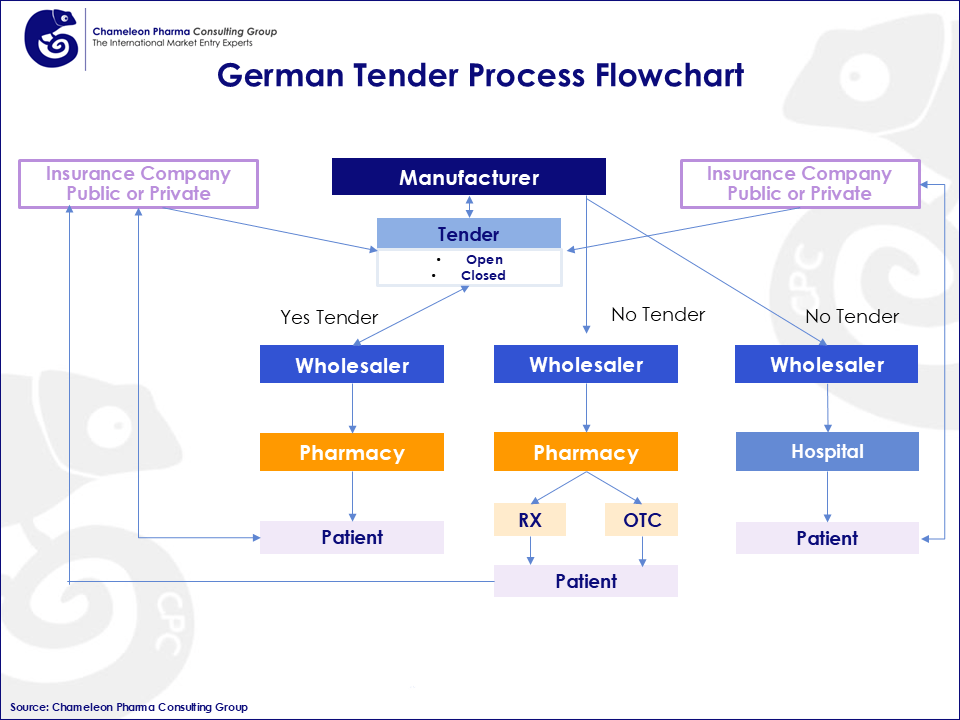
Figure 1: Flowchart of German Pharma Tender Process
Legal background & regulatory framework for discount contracts in Germany
The legal and regulatory framework for pricing and discount (rebate) contracts on Rx medicines is governed by §130 to §131 of Social Code Book V (SGB V). These provisions establish mandatory and optional mechanisms designed to reduce expenditures for the statutory health insurance system (GKV) and ultimately for the patient.
- 130 SGB V regulates the basic structure of mandatory manufacturer rebates
- 130a SGB V outlines additional discounts, especially for generics and off-patent drugs.
- 130c SGB V For high cost medicines, allows for special pricing agreements.
- 131 SGB V supports cost-containment by enabling price freezes and other restrictive pricing measures.
These laws apply exclusively to prescription drugs and are distinct from pricing rules for over-the-counter (OTC) products. Together, they form the foundation for discount contracts (Rabattverträge), which are central to how pharma products are reimbursed and supplied under the public healthcare system.

Figure 2: Overview of the legal background of discount contracts in Germany
Value Chain & Regulated Margins of Rx Drugs in Germany
Prescription (Rx) drug pricing follows a regulated value chain starting with the manufacturer’s ex-factory price (APU). Wholesalers and pharmacies add fixed margins, leading to the retail price towards the patient which is reimbursed by health insurers minus possible co-payments. Manufacturers are also required to grant mandatory discounts, the level of which depends on the product type, and may offer additional rebates through negotiated contracts. These discounts reduce the manufacturer’s net revenue but do not affect the publicly listed retail price.

Figure 3: Flowchart Of the Value Chain & Regulated Margins of Rx Drugs in Germany
What would be the strategic pricing for your products to enter the German Pharma Tender market? CPC would be happy to provide you with a straightforward calculation tool. Contact us for more details!
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




