Feature picture: Photo by Diana Polekhina on Unsplash
Get The Important Insights!
Despite the abundant number of players, there is still a way to dive into the US’ gigantic market. You can start by understanding their easy requirements for food supplements.
Market Trends
In the US, the food supplements market has been benefiting from the growing trend on creating a healthy lifestyle. Recent consumer awareness to meet their nutritional needs, order to prevent potential diseases, and mitigate any problems caused by sedentarism and non-optimal eating habits are helping the market to skyrocket.
These new consumer habits together with the government regulatory rollback and incentives have accelerated the growth and competition in the United States. The US market went from having 4.000 unique products to almost 80.000 different products available to consumers. Right now, the USA is the single largest contributor to global sales of food supplements and is forecasted to reach more than USD 45 bil. in sales by 2035.
Why is the regulatory process driving the market in the US?
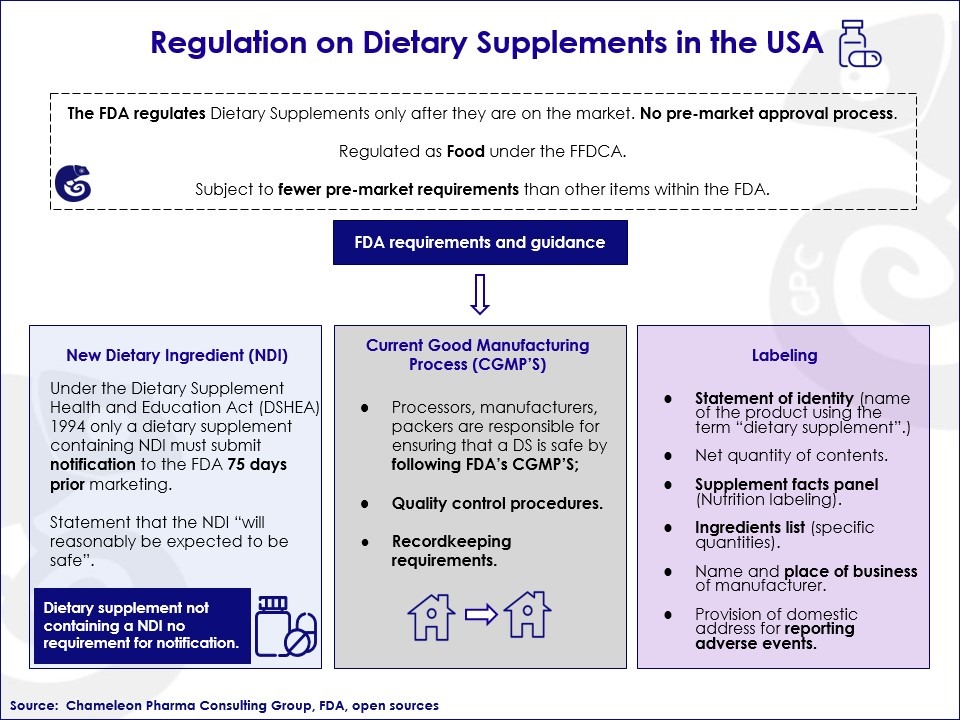
According to the US FDA definition, dietary supplement products and ingredients are any vitamins, minerals, herbs, amino acids, or dietary substances for use by humans to supplement their diet.
Unlike other drugs, dietary supplements are regulated as food under the Federal Food, Drug, and Cosmetic Act (FFDCA). This means that the US FDA does not take regulatory actions before the products are on the market.
Manufacturer and Distributors Responsibilities in the USA
US manufacturers and distributors have the initial responsibility to ensure the safety standards of their dietary supplements. The US FDA has the authority to enforce the law to protect consumers only if these healthcare products have been adulterated, misbranded, or are in violation of federal law.
This makes it easy for companies that want to register their vitamins, minerals, and food supplements because they must simply comply with these procedures to enter the market:
Infographic: Regulation on Dietary Supplements in the USA
Apart from these US FDA guiding requirements to ensure the safety of food supplements, there are no mandatory regulations. This makes it easy to enter the market if you have the right inside knowledge of the US market. Therefore, if you are considering entering the US vitamins, minerals, and food supplements market, Chameleon Pharma Consulting Group is here to support you with the initial regulatory process and following systematic identification of the right local partner and ideal product. Just contact us!




