Photo by Lance Reis on Unsplash
The compounding pharmacy market in Mexico is an evolving sector, experiencing growth in response to increased demand for personalized medication, along with governmental support in maintaining individual’s accessibility to specialized needs. Mexico’s compounding pharmacy market is expected to reach approximately 1.893 Billion USD by 2030, with a CAGR of 25%. This growth reflects the country’s focus on patient-centered healthcare, with a strong market presence in customized medications designed for specific health conditions.
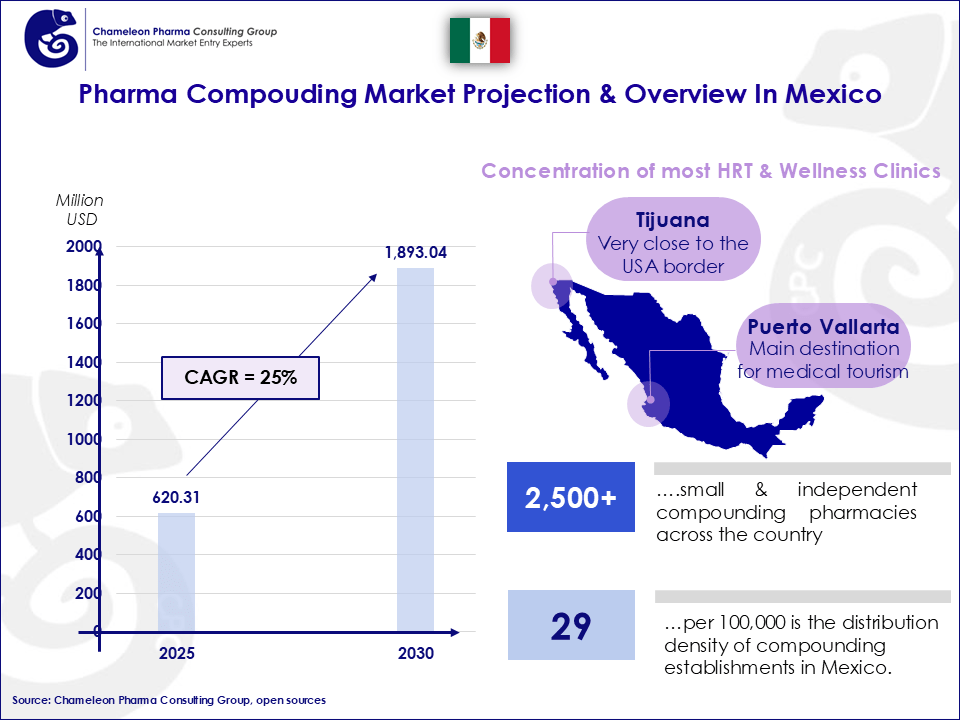
Figure 1: Mexico’s Pharma Compounding Market Overview
Key market insights and competition landscape of Latin America
The compounding pharmacy market for Mexico is growing in relation to the demand for personalized medicine in the country. One of the contribution factors is the increase in governmental support in maintaining individual accessibility to specialized needs. The CAGR of 25% is excellent, reflecting strong and sustained market momentum.
Other key market growth drivers include:
- The Aging Population and Chronic Diseases: The aging population is more vulnerable to chronic diseases, which frequently necessitate customized drugs. Compounded drugs can provide solutions for geriatric patients with special demands.
- Regulatory Support and Quality Standards: Regulatory agencies have set criteria to ensure the safety, efficacy, and quality of compounded drugs. Such assistance boosts market growth by instilling trust in healthcare professionals and patients.
- Advances in technology and pharma compounding techniques have increased the precision and safety of compounding procedures.
- Rising Knowledge and Education: Growing awareness of the benefits of compounding pharmacies among healthcare professionals and patients has helped market growth.
Compared to other countries in Latin America (Brazil, Chile, Colombia, Argentina), Mexico is ranked the highest in the number of compounding pharmacies available in the country. Furthermore, in Mexico, the segment of compounded hormones is more developed than in other Latin American countries.
Mexico has an active compounding pharmacy market, particularly for dermatology and hormone replacement therapies. There are around 37,019 compounding pharmacies and hospitals across the country, which means about 29 facilities are available for every 100,000 people.
Mexico’s regulatory landscape regarding compounding sector
Compounded medicines are strictly regulated in Mexico.
- The regulations only allow certified pharmacists and physicians to make the products at a registered facility.
- There is a list of controlled substances that the pharmacists need to be careful of when compounding the products.
- They need to follow specific procedures, announcements and measurements. The pharmacists must record the amount they have used in the products, as well as the amount they have wasted after compounding the finished products.
As compounded medicines are strictly regulated in Mexico by COFEPRIS it can sometimes become difficult for patients to receive their medicine. For example, in case the products are not available in Mexico, the patients have to submit a request to COFEPRIS (Mexico ministry of Health) to be able to get the medicines from outside (Name-Patient system).
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!