Photo by Anthony Tan on Unsplash
France remains a central reference point in Europe for tightly regulated pharma compounding and patient-specific therapies. Supported by a strong public healthcare system and recurring supply gaps, demand is primarily driven by hospitals and authorized pharmacies rather than on a commercial scale. Looking toward 2040, France’s pharma compounding market is projected to reach approximately $657.7 million, sustained by a CAGR of 4.6%.
France’s Pharma Compounding Market: The Big Picture
France’s pharma compounding market occupies a clearly defined niche within one of Europe’s most structured healthcare systems. Personalized medicine is treated primarily as a clinical necessity, addressing unmet patient needs when authorized medicines are unavailable, unsuitable, or temporarily out of supply. Unlike more liberal markets such as the USA, France has not allowed compounding to develop into a volume-driven commercial activity.
Activity is concentrated in hospital pharmacies (PUI, pharmacies à usage intérieur) and a limited number of authorized community pharmacies, tying demand closely to public health priorities rather than consumer trends.
Market Drivers: Why Compounding Remains Relevant in France
The relevance of personalized medicine in France is underpinned by a small number of structural drivers:
- Recurrent shortages of industrially manufactured medicines
- Pediatric, geriatric, and rare disease requirements
- The need for patient-specific dosing and formulations in the therapeutical areas of hormone, weight loss, anti-aging, and oncology treatment.
These factors position compounding as a responsive healthcare function rather than a growth market.
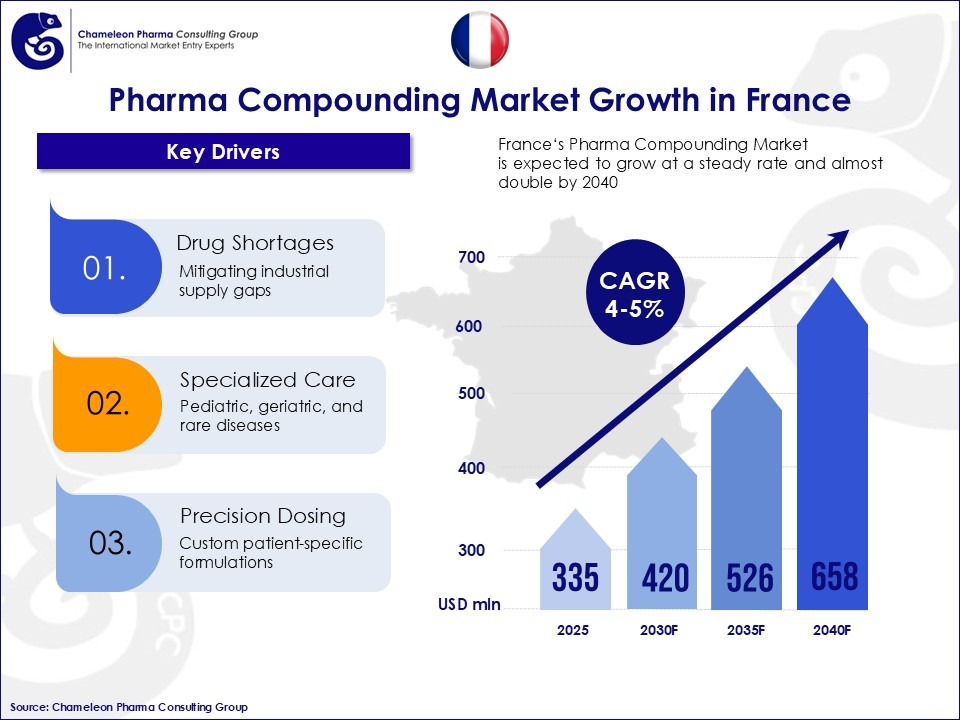
Figure 1: France’s Pharma Compounding Market Overview
French Regulatory Environment: A Highly Controlled Framework
France applies one of the most stringent regulatory frameworks for pharma compounding in Europe. Oversight is led by the Agence nationale de sécurité du médicament et des produits de santé (ANSM) together with regional health authorities.
The framework includes strict authorization and inspection of facilities, mandatory compliance with national good preparation practices (BPP), clear differentiation between magistral, officinal, and industrial medicines, and tight controls on pricing and reimbursement. While this limits commercial flexibility, it ensures high standards of quality, traceability, and patient safety.
The French Pharma Compounding Market Outlook
Compounding will be further centralized within specialized hospital units and supported by digital prescribing across the French pharmacy network, standardized quality systems, and coordinated supply management.
Its long-term value will lie in supporting system resilience, managing shortages, and enabling personalized therapies within the public healthcare infrastructure.
What Comes Next
For international and industrial players, France offers selective opportunities, such as industrial partnerships. These lie primarily in supplying high-quality active ingredients, excipients, technologies, and quality systems, as well as partnering with hospital networks and authorized pharmacies.
On the other hand, the name patient business is possible, and legally feasible, however, under strict conditions. Our CPC regulatory experts can guide your company through the regulatory barriers in the French market and support the compliant market entry in France.
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, Pharmacy compounding segment and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




