Photo by Dimitri Iakymuk on Unsplashed
France’s Pharma and OTC market is entering a decade of accelerated growth, powered by innovation in specialty drugs, rising self-care habits, and the rapid expansion of digital health channels.
France’s Healthcare Market Today: Set to Double in Size by 2040
France has one of the largest healthcare markets in Europe, built on strong public investment and broad access to medical services. Today, the sector is evolving as long-term trends reshape how medicines are distributed and used. These changes are opening new opportunities for growth and ensuring the French market remains a key player in the global pharma industry.
Prescription medicines remain the primary revenue driver for specialty and chronic care, including generics favoured for cost-effectiveness and reimbursement, while Over-The-Counter medicines meet preventive and wellness needs, with adoption rising through digital channels and e-pharmacies for convenient access
The combined market for Rx and OTC products is expected to nearly double, growing from USD 62.3 billion in 2030 to USD 124.2 billion in 2040, a CAGR of 7.1 %.
Key Market Growth Drivers in France
The healthcare market in France is set to nearly double by 2040, driven by several important factors. The main drivers are:
- Self–care adoption: There is a rising consumer awareness and interest in personal wellbeing amongst the French population.
- Ageing population: Around 26 % of the French population is over 60 years old a share expected to rise significantly by 2040, driving higher demand for Rx and OTC products
- Innovation in Rx: The increasing incidence of conditions such as cardiovascular diseases, diabetes, and respiratory disorders boosts the need for both prescription therapies and preventive OTC solutions.
- Healthcare expenditure: France maintains one of Europe’s highest per-capita healthcare expenditures, ensuring strong funding for both prescription treatments and OTC products.
- Skilled workforce: A well-trained pool of pharmacists, clinicians, and medical professionals strengthens engagement with both Rx and OTC consumers.
- Digital health & e-prescriptions: The growth of licensed online pharmacies and gradual adoption of e-prescribing improves accessibility as enhanced by the French government on their Digital Health Roadmap initiative.
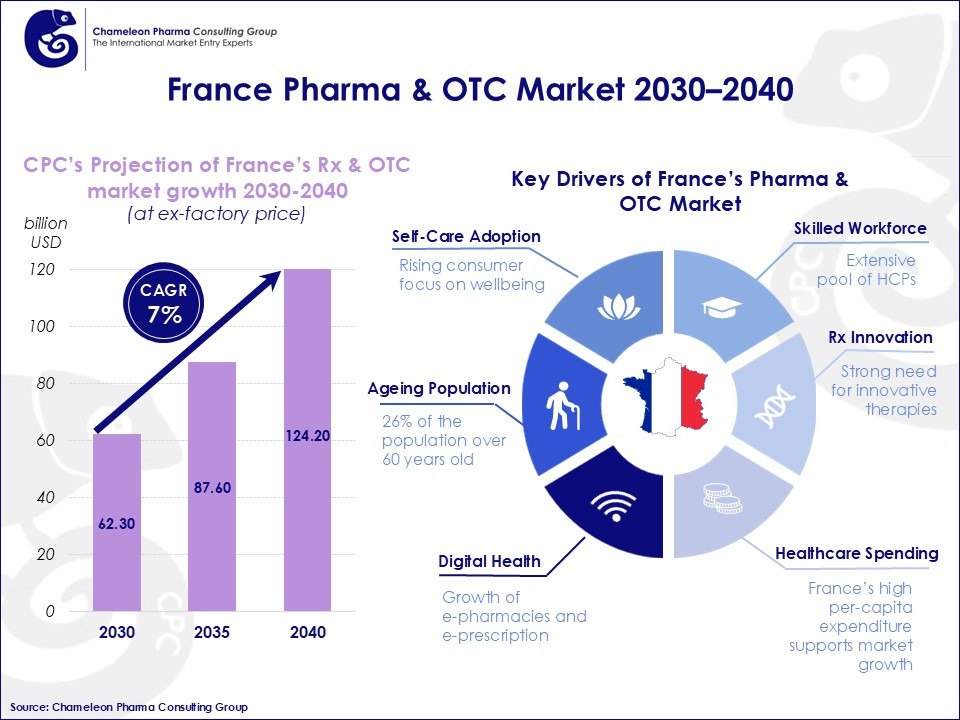
Figure 1: Overview of France’s Pharma & OTC market data in 2030-2040 with key drivers shaping the market
The French Regulatory Landscape
France maintains a solid regulatory framework balancing patient safety, medicine access, and cost control. Important aspects include:
- OTC Registration and Distribution Controls: Most OTC medicines are sold behind the counter with pharmacist guidance.
- Structured Pricing and Reimbursement: The National Agency for the Safety of Medicines and Health Products (ANSM) plays a central role in pricing and reimbursement policies.
- Accelerated Approval for Innovative Therapies: Government initiatives support faster patient access for breakthrough treatments and digital health solutions.
How CPC could assist you in French market entry strategy
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




