Photo by David Restrepo on Unsplash
Colombia is fast becoming a focal point in Latin America for the growth of compounding pharmacies and personalized medicine. With high unmet demand and strong health infrastructure, the market presents unique opportunities thanks to rising demand from hospitals, specialty pharmacies, and wellness clinics.
Colombian Compounding Market Outlook and Size
Colombia’s compounding pharma market is forecasted to grow substantially, from USD 142.1 million in 2030 to USD 473.8 million by 2040, marking a CAGR of 12.8%. This expansion is fueled by increasing interest in customized therapies and demand for treatments not available in standardized commercial formats. The country ranks as the 3rd largest compounding pharmacy market in Latin America after Mexico and Brazil, with total OTC + Pharma sales projected to exceed USD 5.82 billion by 2030.
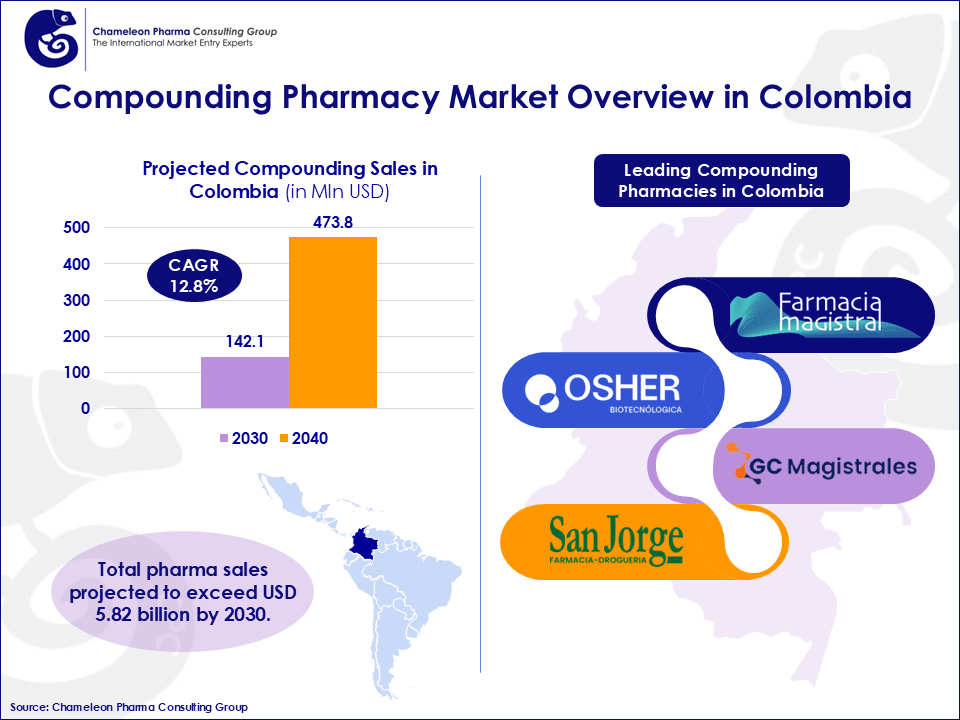
Figure 1: Compounding Pharmacy Market Overview in Colombia
Compounding Sectors and Demand Drivers in Colombia
Colombia’s market for compounding preparations is expanding. One of the major demand drivers is the need for personalized, non-commercially available medicines, particularly for niche therapeutic needs in areas such as hormone replacement therapy (HRT), gynecology, pediatrics, dermatology and anti-aging solutions. These formulations are tailored to individual patients, often based on genetic profiles or real-time health data.
Several key market dynamics underscore Colombia’s potential:
- A significant portion of doctors and patients continue to import compounded medicines from other countries due to insufficient domestic supply.
- The government has fostered open data policies and is encouraging digital integration for better access to health services across remote areas.
- Providers like Fagron are enabling sterile compounding solutions that align with Colombia’s high safety standards.
These factors collectively illustrate why Colombia is one of the leading countries in the region leveraging technology to expand personalized treatment across its diverse geographic landscape.
Regulatory Environment and Pathways for Compounded Medications in Colombia
Colombia maintains a flexible yet solid regulatory framework to support the growing demand for compounding. Compounded medications are regulated by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), ensuring compliance with Good Manufacturing Practices (GMP) and Buenas Prácticas de Elaboración (BPE).
- Compounded medications do not require a marketing authorization (MA) if they are prepared for a specific patient and meet INVIMA’s compounding guidelines.
- Registered drugs from highly regulated markets like the U.S. or EU may benefit from fast track registration process.
- Pharmacies involved in compounding must undergo GMP/BPE certification, including onsite or hybrid inspections, to confirm quality and safety standards.
Additionally, finished compounded products can be imported, but only under strict conditions, such as absence of therapeutic alternatives in Colombia and adherence to special import procedures.
Future Outlook of Colombian Pharma Compounding
Colombia’s compounding pharma sector is undergoing rapid transformation, fueled by healthcare digitalization, strong clinical demand, and a supportive regulatory framework. With significant opportunities in personalized and specialty medicine, an expanding patient base, and clear regulatory pathways, Colombia offers fertile ground for international investment and innovation. As unmet needs remain high, the market is primed for companies skilled in regulatory navigation and advanced compounding technologies.
Chameleon Pharma Consulting Group (CPC) has over 20 years of experience in supporting Pharma, OTC, Medical Devices, Phyto, Pharmacy compounding segment and Aesthetic Medicine companies. Having established own offices & local hubs across Latin America, Europe, Asia, the US/Canada, the Middle East, and the CEE/CIS regions is another advantage of CPC. With this local network and expertise gained from 300+ international projects and a team of 25 experts we offer our clients:
- Business Development, M&A, and Due Diligence
- Market Entry & Expansion: Systematic product and country analysis, market reports
- Strategic Partnering: Identifying local partners, acquisitions, or setting up own offices
- Regulatory & Registration: for drugs, MD, Derma, Aesthetic Medicine, etc.
- Market Authorization & Compliance: Holding MAs, conducting pharmacovigilance
- Quality & Certification: GMP certification, pre-GMP audits
Contact us today for your individual request at service@chameleon-pharma.com!




